The figure shows a PV diagram for 8.3 g of nitrogen gas in a sealed container.The temperature of state 1 is 79°C.What are (a) pressure p1 and (b) temperature T2? 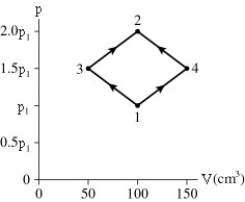
A) (a) 86 atm (b) 700°C
B) (a) 19 atm (b) 700°C
C) (a) 86 atm (b) 160°C
D) (a) 19 atm (b) 160°C
Correct Answer:
Verified
Q29: A person consumes a large meal containing
Q30: A 7 kg sample of mercury is
Q31: As 771.0 kg copper bar is put
Q32: A heat conducting rod,0.90 m long,is made
Q33: An object having an emissivity 0.725 radiates
Q35: A glass beaker of unknown mass contains
Q36: A container with rigid walls is filled
Q37: At what temperature would the average thermal
Q38: Heat is added to a 2.0 kg
Q39: The figure shows a 50 kg lead
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents