The figure shows a pV diagram for 0.0066 mol of gas that undergoes the process 1 → 2 → 3. What is the pressure p2. The ideal gas constant is R = 8.314 J/mol ∙ K = 0.0821 L ∙ atm/mol ∙ K. 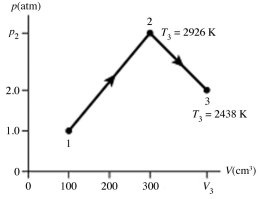
A) 5.3 atm
B) 5.3 × 105 atm
C) 16 atm
D) 1.6 × 106 atm
Correct Answer:
Verified
Q1: When an ideal gas increases in volume
Q3: A container of ideal gas has a
Q4: When a fixed amount of ideal gas
Q5: The figure shows a pV diagram for
Q6: An ideal gas in a balloon is
Q7: The figure shows a pV diagram for
Q7: When a fixed amount of ideal gas
Q8: A steel container,equipped with a piston,contains 21
Q8: The process shown in the pV diagram
Q15: The figure (not to scale) shows a
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents