A diatomic has a moment of inertia of 7.73 × 10-45 kg∙ m2. What is its rotational energy in the quantum state characterized by l = 2? (h = 6.626 × 10-34 J ∙ s, 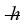 = 1.055 × 10-34 J ∙ s, 1 eV = 1.60 × 10-19 J)
= 1.055 × 10-34 J ∙ s, 1 eV = 1.60 × 10-19 J)
A) 22.7 µeV
B) 27.0 µeV
C) 587 µeV
D) 72.2 µeV
E) 87.1 µeV
Correct Answer:
Verified
Q1: A rotating diatomic molecule has rotational quantum
Q6: A certain diatomic molecule emits a photon
Q7: A diatomic molecule has 2.6 × 10-5
Q7: A rotating diatomic molecule in its l
Q8: Estimate the rotational energy (in eV) for
Q8: In a p-type semiconductor,a hole is
A) a
Q10: The spacing of the atoms (treated as
Q11: A diatomic molecule has 18 × 10-5
Q13: Ionic bonding is due to
A) the sharing
Q19: A p-type semiconductor has a net positive
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents