Two deuterium nuclei, 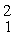 H, fuse to produce a tritium nucleus,
H, fuse to produce a tritium nucleus, 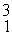
H, and a hydrogen nucleus. A neutral deuterium atom has a mass of 2.014102 u; a neutral tritium atom has a mass of 3.016049 u; a neutral hydrogen atom has a mass of 1.007825 u; a neutron has a mass of 1.008665 u; and a proton has a mass of 1.007276 u. How much energy is released in the process? (1 u = 931.494 MeV/c2)
A) 3.03 MeV
B) 3.53 MeV
C) 4.03 MeV
D) 4.53 MeV
E) 6.58 MeV
Correct Answer:
Verified
Q79: How much energy is released in the
Q80: An ancient rock is found to contain
Q82: A 70-kg laboratory technician absorbs 2.9 mJ
Q83: The reaction 2H + 2H →? 3H
Q83: A certain isotope has a half-life of
Q84: The radioactive nuclei 60Co is widely used
Q86: A 57-kg researcher absorbs 6.3 × 108
Q88: Consider the fusion reaction Q89: The maximum permissible workday dose for occupational Q89: Two deuterium nuclei, ![]()
![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents