One of the buffers that contribute to pH stability in human blood is carbonic acid (H2CO3) . Carbonic acid is a weak acid that, when placed in an aqueous solution, dissociates into a bicarbonate ion (HCO3−) and a hydrogen ion (H+) , as noted below. 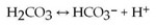 If the pH of blood increases, one would expect ________.
If the pH of blood increases, one would expect ________.
A) a decrease in the concentration of H2CO3 and an increase in the concentration of HCO3−
B) an increase in the concentration of H2CO3 and a decrease in the concentration of HCO3−
C) a decrease in the concentration of HCO3− and an increase in the concentration of H+
D) an increase in the concentration of HCO3− and a decrease in the concentration of OH−
E) a decrease in the concentration of HCO3− and an increase in the concentration of H2CO3 and H+
Correct Answer:
Verified
Q7: Which type of bond must be broken
Q16: In a single molecule of water, two
Q20: A covalent chemical bond is one in
Q21: The partial negative charge in a molecule
Q22: Based on your knowledge of the polarity
Q23: You need to represent a molecule to
Q24: You have two beakers. One contains pure
Q26: Why does ice float in liquid water?
A)
Q27: The partial negative charge at one end
Q30: Which of the following is a property
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents