Based on your knowledge of the polarity of water molecules, the solute molecule depicted here is most likely ________. 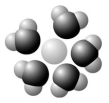
A) positively charged
B) negatively charged
C) without charge
D) hydrophobic
E) nonpolar
Correct Answer:
Verified
Q18: From its atomic number of 15, it
Q19: Q20: A covalent chemical bond is one in Q21: The partial negative charge in a molecule Q23: You need to represent a molecule to Q24: You have two beakers. One contains pure Q25: One of the buffers that contribute to Q26: Why does ice float in liquid water? Q27: The partial negative charge at one end Q46: Refer to the following figure to answer![]()
A)
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents