Consider the following reaction at equilibrium: 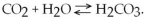 What would be the effect of adding additional H2CO3?
What would be the effect of adding additional H2CO3?
A) It would drive the equilibrium dynamics to the right.
B) It would drive the equilibrium dynamics to the left.
C) Nothing would happen, because the reactants and products are in equilibrium.
D) The amounts of CO2 and H2O would decrease.
Correct Answer:
Verified
Q11: Why is carbon so important in biology?
A)
Q49: Which of the following correctly describes a
Q50: During chemical evolution, which of the following
Q52: Which of the functional groups below acts
Q53: Why are some reactions exothermic?
A) The products
Q54: Which of the following is TRUE of
Q55: Why do chemical reactions tend to speed
Q56: The first chemicals that provided potential energy
Q57: _ atoms give organic molecules their overall
Q58: Stanley Miller's 1953 experiments supported the hypothesis
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents