What is the geometry of the N in the following molecule? 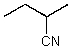
A) tetrahedral
B) trigonal pyramidal
C) linear
D) bent
E) trigonal planar
Correct Answer:
Verified
Q18: Which of these substances contain both covalent
Q115: In which molecule(s)can the molecular geometry be
Q118: The bond angle for the C-S-C
Q122: Identify the atomic orbitals in the C-O
Q124: Which molecule would be linear? (In each
Q125: The following electron configuration represents: 
Q134: The bond angle for the C-C-N
Q141: Draw all isomers of C4H8,using bond-line formulas.
Q144: Draw all the isomers of C4H9Br,using bond-line
Q147: Organic compounds were originally defined as compounds
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents