Identify the atomic orbitals in the C-O sigma bond in acetone. 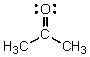
A) (2sp2,2sp2)
B) (2sp3,2sp3)
C) (2sp,2sp)
D) (2p,2p)
E) (2sp,1s)
Correct Answer:
Verified
Q18: Which of these substances contain both covalent
Q118: The bond angle for the C-S-C
Q120: What is the geometry of the N
Q125: The following electron configuration represents: 
Q134: The bond angle for the C-C-N
Q141: Draw all isomers of C4H8,using bond-line formulas.
Q143: Draw all the isomers of C4H10O,using bond-line
Q144: Draw all the isomers of C4H9Br,using bond-line
Q147: Organic compounds were originally defined as compounds
Q153: Constitutional isomers differ in the _.
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents