Multiple Choice
Which of the following reactions would have the smallest energy of activation?
A) 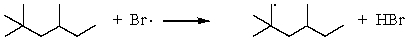
B) 
C) 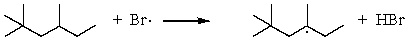
D) 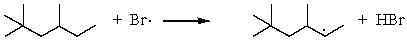
E) 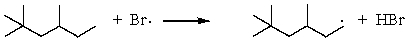
Correct Answer:
Verified
Related Questions
Q13: In the presence of light at 25
Q17: Hydrogen atom abstraction from which position would
Q18: The
Q20: An alternate mechanism for the chlorination
Q22: Which of the following reactions would
Q24: Which of the following statements is true
Q25: For which of the following gas-phase
Q26: Which of the following reactions would
Q27: Select the structure of the major product
Q28: What feature would you expect to see
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents