Two cylinders are connected by a small tube which has a needle valve in the middle.One cylinder contains 4He gas,and the other cylinder contains CO2 gas.The gases are at the same temperature,pressure and volume.The lids that seal the gases in are free to move up or down.When the valve is opened,what happens to the volume in each cylinder? 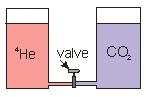
A) The volumes do not change.
B) The CO2 volume decreases and the 4He increases.
C) The CO2 volume increases and the 4He decreases.
D) What happens depends on the initial temperature.
E) What happens depends on the initial pressure.
Correct Answer:
Verified
Q66: A room measures 3 m
Q67: When the temperature of an ideal gas
Q68: A room measures 3 m
Q69: If the rms speed of oxygen molecules
Q70: A 1 L container contains O2
Q72: Use the following to answer the question:
Q73: On the basis of the kinetic theory
Q74: If the rms speed of nitrogen molecules
Q75: Use the following to answer the question:
Q76: A device used to measure the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents