Use the following to answer question: 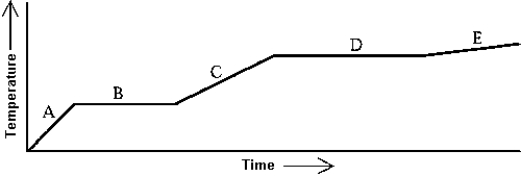
-Heat is added to a substance at a constant rate.The substance starts as a solid and is melted; the liquid is heated and vaporized; finally,the vapor is heated at constant volume.This process is shown in the graph.The specific heat at constant volume of the vapor can be found by
A) multiplying the length of B (in seconds) by the rate at which heat is added,and dividing by the mass of the substance.
B) multiplying the length of D (in seconds) by the rate at which heat is added,and dividing by the mass of the substance.
C) dividing the rate at which heat is added by the product of the slope of A and the mass of the substance.
D) dividing the rate at which heat is added by the product of the slope of C and the mass of the substance.
E) dividing the rate at which heat is added by the product of the slope of E and the mass of the substance.
Correct Answer:
Verified
Q15: The specific heat of a substance is
Q16: A 1.0-kg piece of marble at 100ºC
Q17: To raise the temperature of a 2.0-kg
Q18: Use the following to answer question:
Q19: A system has a heat capacity of
Q21: A 4-kg mass of metal of
Q22: A small lake has a surface
Q23: A container contains a 200 mL of
Q24: You add 50 g of ice cubes
Q25: Use the following to answer question:
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents