Use the following to answer the question: 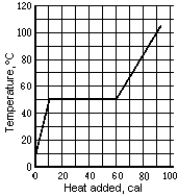
-The graph shows the temperature of a 1.0-g sample of material as heat is added to it.The material is initially a solid at 10ºC.The pressure remains constant,and there is no chemical change.The specific heat of the liquid phase is
A) 0.84 cal/g · Cº
B) 0.25 cal/g · Cº
C) 1.6 cal/g · Cº
D) 1.7 cal/g · Cº
E) None of these is correct.
Correct Answer:
Verified
Q38: If 100 g of steam at
Q39: Some water is poured into some ice.What
Q40: On a hot summer day,water collects on
Q41: Use the following to answer the question:
Q42: Suppose you do 75 kJ of work
Q44: In a certain thermodynamic process,1000 cal of
Q45: Besides Joule's classic experiment,another way of demonstrating
Q46: The first law of thermodynamics has as
Q47: A small water reactor recently installed at
Q48: Use the following to answer the question:
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents