Use the following to answer the question: 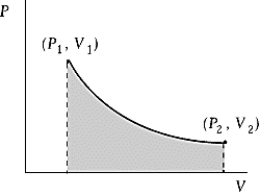
-An ideal gas initially at 100ºC and pressure P1 = 250 kPa occupies a volume V1 = 4.5 L.It undergoes a quasistatic,isothermal expansion until its pressure is reduced to 150 kPa.How much heat enters the gas during this process? R = 8.314 J/mol·K = 8.206 L·atm/mol·K.
A) 116 J
B) 320 J
C) 575 J
D) 640 J
E) 850 J
Correct Answer:
Verified
Q72: Use the following to answer the
Q73: Use the following to answer the question:
Q74: The equation of state for a certain
Q75: Use the following to answer the
Q76: From the measured molar heat capacities and
Q78: An ideal gas is heated so that
Q79: The work done by an ideal
Q80: A liquid is irregularly stirred in a
Q81: The fact that most solids have molar
Q82: The pressure of a mass of
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents