If you measure the angular momentum of an electron in units of 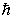 ,you find that the angular momentum is quantized to the value _______ units and that its component along any direction can have only the _______ values ranging from -
,you find that the angular momentum is quantized to the value _______ units and that its component along any direction can have only the _______ values ranging from - 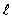 to +
to + 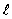 units.
units.
A) 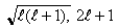
B) 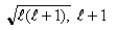
C) 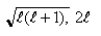
D) 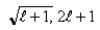
E) 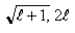
Correct Answer:
Verified
Q37: Bohr's quantum condition on electron orbits
Q38: What is the ratio of the radius
Q39: What is the difference in wavelength between
Q40: The energy of the nth level in
Q41: The possible orbital quantum numbers of an
Q43: If the angular momentum is characterized by
Q44: Use the following figure for the next
Q45: If the angular momentum is characterized by
Q46: Consider a beryllium (Z = 4)ion with
Q47: A hydrogen atom that has an electron
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents