Silicon is one of the Group IV semiconductors and has an energy gap of 1.12 eV.Generally only metals have highly shiny surface,yet as shown in the figure,silicon appears to reflect like a metal.This is because 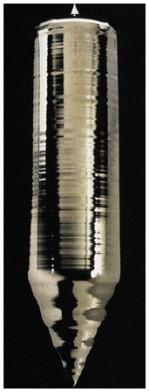
A) silicon is a good semiconductor with a well defined energy gap.
B) visible light has energies in the range 2-3 eV which is much larger than the energy gap allowing electrons to be promoted from the valence band to the conduction band.
C) the surface of silicon is smooth making it reflect like a metal.
D) silicon can be grown in very high purity.It is the impurities that make a semiconductor dull looking.
E) None of these is correct.
Correct Answer:
Verified
Q40: The resistivity of nichrome at 20ºC
Q41: If you apply a voltage of 3
Q42: One of the principal advantages of the
Q43: Reverse biasing of a pn junction tends
Q44: A metal is a good conductor because
Q46: You place sodium and copper in
Q47: A semiconductor for which it is true
Q48: Insulators are poor conductors of electricity because
A)there
Q49: Use the following to answer the question:
Q50: A Josephson junction is a junction of
A)two
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents