The total angular momentum of a hydrogen atom in a given excited state has the quantum number j = 5/2. The orbital angular-momentum quantum number 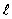 must have the values
must have the values
A) 0 or 1
B) 1 or 2
C) 2 or 3
D) 3 or 4
E) 5 or 6
Correct Answer:
Verified
Q70: The hydrogen-like elements (valence +1)exhibit a splitting
Q72: You will never find an electron in
Q78: Which of the following transitions are allowed
Q87: The electron configuration of nitrogen (Z =
Q91: A hydrogen atom is in the state
Q92: The number of electrons in the M
Q93: The number of electrons in the L
Q95: The energy of an electron in an
Q97: The d state of an electronic configuration
Q101: ![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents