A hydrogen atom is in the state n = 4, 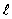 = 3. The possible values of j are
= 3. The possible values of j are
A) 1/2 and 0
B) 3/2 and 1/2
C) 5/2 and 3/2
D) 7/2 and 5/2
E) 9/2 and 7/2
Correct Answer:
Verified
Q81: The electron configuration of neon (Z =
Q86: The total angular momentum is 
Q87: The electron configuration of nitrogen (Z =
Q88: The number of oxygen's eight electrons that
Q89: A hydrogen atom is in the state
Q90: Z for the element whose electronic configuration
Q92: The number of electrons in the M
Q93: The number of electrons in the L
Q95: The energy of an electron in an
Q96: The total angular momentum of a hydrogen
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents