A hydrogen atom is in the state n = 4, 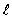 = 3. The two spectral states due to spin-orbit coupling are
= 3. The two spectral states due to spin-orbit coupling are
A) 3d7/2 and 3d5/2
B) 4f7/2 and 4f5/2
C) 4f5/2 and 4f3/2
D) 3d5/2 and 4d3/2
E) 4d7/2 and 4d5/2
Correct Answer:
Verified
Q76: It was _ who showed that the
Q81: The electron configuration of neon (Z =
Q86: The total angular momentum is 
Q87: The electron configuration of nitrogen (Z =
Q88: The number of oxygen's eight electrons that
Q90: Z for the element whose electronic configuration
Q91: The Pauli exclusion principle states that
Q91: A hydrogen atom is in the state
Q92: The number of electrons in the M
Q93: The number of electrons in the L
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents