Which of the following statements about the reaction shown below is true? 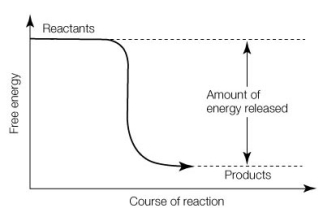
A) It is an endergonic reaction.
B) The reactants have less energy than the products.
C) G is negative.
D) The reaction can be reversed without the addition of energy.
E) It is an anabolic reaction.
Correct Answer:
Verified
Q104: ATP is necessary for the conversion of
Q106: Coenzymes and cofactors, as well as prosthetic
Q107: Ascorbic acid, found in citrus fruits, acts
Q107: When an enzyme is heated until its
Q108: Which of the following contributes to the
Q110: Which of the following are characteristics of
Q111: Use the following to answer questions:
In the
Q112: Which of the following represents an
Q113: Enzymes are biological catalysts and function by
A)
Q114: Before ATP is split into ADP and
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents