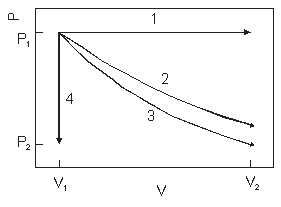
 The diagram above show the state of an ideal gas going from (V1,P1) to a final state. Which path best represents adiabatic expansion?
The diagram above show the state of an ideal gas going from (V1,P1) to a final state. Which path best represents adiabatic expansion?
A) 1
B) 2
C) 3
D) 4
E) none of the paths
Correct Answer:
Verified
Q65: Use the following to answer the question:
Q72: Use the following to answer the
Q73: Use the following to answer the question:
Q81: The fact that most solids have molar
Q82: The pressure of a mass of
Q83: At a particular point on a PV
Q97: The equipartition theorem
A)fails to explain the fact
Q98: In a system composed of gas contained
Q99: The difference in the molar heat capacity
Q100: A system is said to go through
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents