What is the hybridization of the N atom in the following molecule? 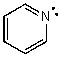
A) s
B) p
C) sp
D) sp2
E) sp3
Correct Answer:
Verified
Q103: The following electron configuration represents: 
Q104: What bond angle is associated with
Q105: Identify the atomic orbitals in the N-O
Q106: The following electron configuration represents: 
Q107: What is the geometry of the C
Q109: Identify the atomic orbital the lone pair
Q110: Which molecule contains an sp-hybridized carbon?
A)HCN
B)CH2=CH2
C)CH3Cl
D)
Q111: What geometry does the methyl cation,CH3+,have?
A)octahedral
B)tetrahedral
C)trigonal planar
D)linear
E)trigonal
Q112: Identify the hybridized orbitals involved in the
Q113: Identify the atomic orbital the lone pair
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents