Identify the atomic orbitals in the N-O sigma bond in the following oxime: 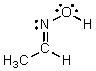
A) (2sp2,2sp2)
B) (2sp3,2sp3)
C) (2sp,2sp)
D) (2sp2,2sp3)
E) (2sp,1s)
Correct Answer:
Verified
Q100: Identify the atomic orbitals in the C-C
Q101: The bond angles for the bold-faced
Q102: The bond angle for the C-C-O
Q103: The following electron configuration represents: 
Q104: What bond angle is associated with
Q106: The following electron configuration represents: 
Q107: What is the geometry of the C
Q108: What is the hybridization of the N
Q109: Identify the atomic orbital the lone pair
Q110: Which molecule contains an sp-hybridized carbon?
A)HCN
B)CH2=CH2
C)CH3Cl
D)
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents