The bond angle for the C-C-O bonds in the following molecule would be expected to be approximately: 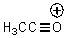
A) 90
B) 109
C) 120
D) 145
E) 180
Correct Answer:
Verified
Q97: How many 2p atomic orbitals from boron
Q98: Identify the atomic orbital the lone pair
Q99: Which is the shortest of the carbon-carbon
Q100: Identify the atomic orbitals in the C-C
Q101: The bond angles for the bold-faced
Q103: The following electron configuration represents: 
Q104: What bond angle is associated with
Q105: Identify the atomic orbitals in the N-O
Q106: The following electron configuration represents: 
Q107: What is the geometry of the C
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents