Consider the graph below,which is a plot of the relative energies of the various conformations of hexane,viewed through the C-2-C-3 bond.The conformations corresponding to the 60o and 300o are: 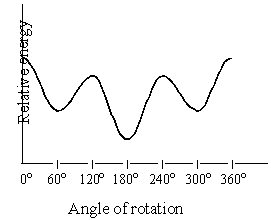
A) eclipsed
B) staggered and gauche
C) staggered and anti
D) more stable than the conformation at 180o
E) none of these choices
Correct Answer:
Verified
Q47: Which staggered Newman projection(s),looking down the C-3-C-4
Q48: Consider the graph below,which is a plot
Q49: The most stable conformation of 2,3-dibromobutane,viewed through
Q50: Which conformation(s)of 1,2-dibromoethane does not illustrate one
Q51: A correct IUPAC name for the following
Q53: The most stable conformation of butane is:
Q54: Which staggered Newman projection(s),looking down the C-2-C-3
Q55: What is the correct IUPAC name for
Q56: The IUPAC name for diisobutylacetylene is
A)2,7-Dimethyl-4-octene
B)2,7-Dimethyl-4-octyne
C)3,6-Dimethyl-4-octyne
D)2,5-Diethyl-3-hexyne
E)2,2,5,5-Tetramethyl-3-hexyne
Q57: The graph below is a plot of
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents