Recalling that benzene has a resonance energy of 152 kJ mol-1 and naphthalene has a resonance energy of 255 kJ mol-1,predict the positions which would be occupied by bromine when phenanthrene (below) undergoes addition of Br2. 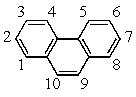
A) 1,2
B) 1,4
C) 3,4
D) 7,8
E) 9,10
Correct Answer:
Verified
Q40: Which is the only one of these
Q41: Which reagent(s)would serve as the basis for
Q42: Which reagent(s)would serve as the basis for
Q43: In the molecular orbital model of
Q44: In the molecular orbital model of benzene,how
Q46: Application of the polygon-and-circle technique reveals that
Q47: Which cyclization(s)should occur with an increase in
Q48: In the molecular orbital model of benzene,the
Q49: Why would 1,3-cyclohexadiene undergo dehydrogenation readily?
A)It is
Q50: Cyclopentadiene is unusually acidic for a hydrocarbon.An
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents