Which cyclization(s) should occur with an increase in pi-electron energy? 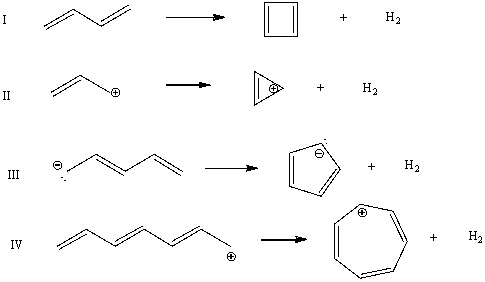
A) I
B) II
C) III
D) IV
E) All of these choices.
Correct Answer:
Verified
Q42: Which reagent(s)would serve as the basis for
Q43: In the molecular orbital model of
Q44: In the molecular orbital model of benzene,how
Q45: Recalling that benzene has a resonance energy
Q46: Application of the polygon-and-circle technique reveals that
Q48: In the molecular orbital model of benzene,the
Q49: Why would 1,3-cyclohexadiene undergo dehydrogenation readily?
A)It is
Q50: Cyclopentadiene is unusually acidic for a hydrocarbon.An
Q51: In the molecular orbital model of
Q52: Which of the following statements about cyclooctatetraene
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents