Consider the oxygen atom in the molecules of furan and tetrahydrofuran.Bearing in mind that furan exhibits aromatic properties,compare the hybridization of oxygen in both species,explaining why it must be different,although at first glance,it may appear identical. 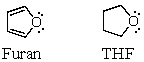
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q176: Draw the structure corresponding to the following
Q177: Briefly explain why the aromatic hydrocarbon azulene,C10H8,possesses
Q178: Cycloheptatriene is found to readily undergo a
Q179: Draw the structure corresponding to the following
Q180: The Lewis structures of both pyridine and
Q181: Draw all significant resonance structures for azulene,C10H8
Q182: Although all bond lengths in benzene are
Q183: Draw all significant resonance structures for the
Q184: Draw all significant resonance structures for pyridine,C6H5N
Q185: The compound illustrated below is found
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents