A Compound Has the Molecular Formula,C6H12O2 14 And Has 20The Most Likely Structure for This Compound Is:
A)I
A compound has the molecular formula,C6H12O2.Its IR spectrum shows a strong absorption band near 1740 cm-1; its 1H NMR spectrum consists of two singlets,at 1.4 and 2.0.The most likely structure for this compound is: 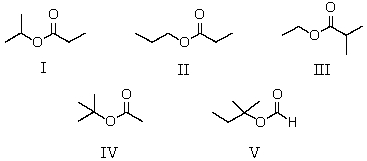
A) I
B) II
C) III
D) IV
E) V
Correct Answer:
Verified
Q40: In which of the following sequences
Q41: Which of the following acids would be
Q42: Which of the following acids would be
Q43: In which of the following are the
Q44: Which of the following acids would have
Q46: Which compound would be most acidic?
A)
Q47: Which of the following would be the
Q48: A compound has the molecular formula
Q49: Which of the following would be the
Q50: Which of the following acids would have
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents