A compound has the molecular formula C8H14O4.Its IR spectrum shows a strong absorption band near 1740 cm-1.Its 1H NMR spectrum consists of: triplet, 1.3
Singlet, 2.6
Quartet, 4.2
The most likely structure for the compound is: 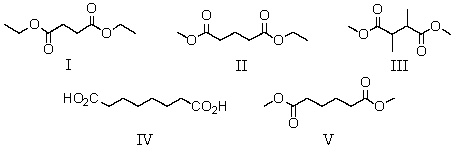
A) I
B) II
C) III
D) IV
E) V
Correct Answer:
Verified
Q43: In which of the following are the
Q44: Which of the following acids would have
Q45: A compound has the molecular formula,C6H12O2.Its
Q46: Which compound would be most acidic?
A)
Q47: Which of the following would be the
Q49: Which of the following would be the
Q50: Which of the following acids would have
Q51: A compound has the molecular formula,C6H12O2.Its
Q52: Which of the following acids would be
Q53: The IR spectrum of a compound exhibits
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents