The free energy of activation, ΔG 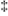 , is defined as:
, is defined as:
A) The average free energy of the product formed.
B) The rate of a chemical reaction in relationship to the concentration of reactant molecules.
C) The energy required to raise the average energy of one mole of reactant to the transition state energy.
D) The amount of energy released by a spontaneous reaction.
E) The lowest point on a free energy diagram.
Correct Answer:
Verified
Q1: All are distinctive features of enzymes EXCEPT:
A)
Q2: All are true for catalysts EXCEPT:
A) They
Q4: Which of the following is true regarding
Q5: The specific site on the enzyme where
Q6: All of the following are true statements
Q7: What reaction would NOT proceed via bimolecular
Q8: All of the following are properties of
Q9: Which of the following statements is true
Q10: Enzymes work by:
A) providing an alternate reaction
Q11: For an enzyme-catalyzed reaction, the initial velocity
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents