System 1 is 3.5 moles of a monatomic ideal gas held at a constant volume of 2.22 L as it undergoes a temperature increase.System 2 is 3.5 moles of a diatomic ideal gas held at a constant volume of 2.22 L as it undergoes a temperature increase equal to the temperature increase of System 1.The pressure increase for system 1 is 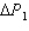 and for System 2 is
and for System 2 is 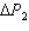 during the temperature increases.What is the ratio of
during the temperature increases.What is the ratio of 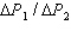 ?
?
A) 1.00
B) 0.600
C) 1.67
D) 2.50
Correct Answer:
Verified
Q18: Heat is applied to an ice-water mixture
Q21: The maximum theoretical thermodynamic efficiency of a
Q29: A cylinder containing an ideal gas has
Q30: An ideal gas at pressure,volume,and temperature,P0,V0,and T0,respectively,is
Q31: How much thermal energy must be added
Q32: A heat engine operating between a pair
Q33: An electrical generating plant operates at
Q36: An electrical generating plant operates at
Q38: According to the second law of thermodynamics,which
Q39: An electrical power plant manages to send
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents