Multiple Choice
Based on the information in the table of standard reduction potentials below,calculate G for the redox reaction of bromine with potassium. 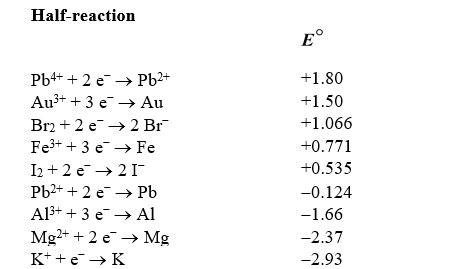
A) "+359 kJ"
B) "-359 kJ"
C) "-1340 kJ"
D) "+1340 kJ"
E) "-771 kJ"
Correct Answer:
Verified
Related Questions
Q61: Calculate Q62: An electronic device requires two 1.50-V Q62: An electronic device requires two 1.50-V Q64: If the potential of a voltaic cell Q65: Which statement does NOT correctly describe Q65: The spontaneous redox reaction in a Q67: In one episode of the 1960s television![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents