There are many reactions involving nitrogen,oxygen,and nitrogen oxides at high temperatures.At 1000.0 K,the following reactions have the given equilibrium constants: N2O4(g) 2 NO2(g) K1 = 1.5 106 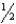 N2(g) + O2(g) NO2(g) K2 = 1.2 10-5
N2(g) + O2(g) NO2(g) K2 = 1.2 10-5
Calculate the theoretical value of the equilibrium constant for N2(g) + 2 O2(g) N2O4(g) .
A) 9.6 10-17
B) 1.6 10-11
C) 6.7 10-7
D) 1.0 1016
E) 36
Correct Answer:
Verified
Q37: Under what conditions are the values of
Q38: Consider the equilibrium,Cl2(g)+ 2 NO(g)
Q39: For the chemical equilibrium aA +
Q40: Consider the following equilibrium: ClF3(g)
Q41: Two students measure the equilibrium constant for
Q43: A series of four equilibrium steps
Q44: For the equilibrium 2 PH3(g)
Q45: Chromic acid is a diprotic acid:
Q46: Which of the following statements regarding the
Q47: Write the expression for the reaction
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents