For the equilibrium (CH3) 3CCl(g) (CH3) 2C = CH2(g) + HCl(g) ,Kp = 3.45 at 500 K.At a given point,the partial pressures of the gases are 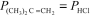 = 0.810 atm and
= 0.810 atm and 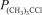 = 0.190 atm.Which of the following statements is true?
= 0.190 atm.Which of the following statements is true?
A) Q < K so the reaction will continue to make more products.
B) Q > K so the reaction will consume products to make more reactants.
C) Q = K so the system is at equilibrium.
D) The value of K will decrease until it is equal to Q.
E) The value of K will increase until it is equal to Q.
Correct Answer:
Verified
Q43: Cylinders of NO gas may contain small
Q59: Which of the following occurs when products
Q73: In a simple equilibrium A +
Q74: Naphthalene undergoes sublimation: C10H8(s)
Q76: Increasing the temperature of an exothermic reaction
Q77: Which of the following occurs when reactants
Q79: Which of the following is the
Q81: For the gas-phase oxidation of sulfur
Q82: For a particular hypothetical reaction,2 A
Q83: For a chemical reaction at equilibrium, which
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents