A chemist is planning to study a chemical equilibrium for which no measured explicit thermodynamic values are available.However,the reaction involves common chemical species.What information could a chemist use to make predictions about the equilibrium?
A) "equilibrium constants for the equilibrium reactions that can be combined to make the new equilibrium"
B) "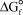 values for the reactants and products of the new reaction"
values for the reactants and products of the new reaction"
C) " Hf and S values for the reactants and products of the new reaction"
D) "only b and c"
E) "all of the above"
Correct Answer:
Verified
Q92: Several groups of general chemistry lab
Q103: As the temperature of a reaction
Q104: A sketch of the free energy
Q105: Using the thermodynamic data below,determine the
Q106: Which of the following figures illustrates best
Q110: What is the value of the
Q111: A student can accept that
Q112: Using the thermodynamic data below,determine the
Q113: As the temperature of an endothermic
Q132: The equilibrium constant for a given reaction
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents