The values of 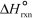 and
and 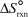 are shown for the following equilibrium reaction.What makes the equilibrium constant larger at low temperature? 2 NO2(g) N2O4(g) H = -58.02 kJ/mol, S =-176.5 J/mol . K
are shown for the following equilibrium reaction.What makes the equilibrium constant larger at low temperature? 2 NO2(g) N2O4(g) H = -58.02 kJ/mol, S =-176.5 J/mol . K
A) The negative value of H is dominant at all temperatures.
B) The negative value of S is dominant at all temperatures.
C) The negative value of S is combined with the small value of T.
D) The negative value of H is combined with the small value of T.
Correct Answer:
Verified
Q84: Chemical equilibrium arises from the _ of
Q96: A qualitative interpretation of the effect
Q127: For the equilibrium 2 OF2(g)
Q129: Given the following two measurements of
Q130: Consider the equilibrium CO(g)+ 2 H2(g)
Q131: Gaseous carbon monoxide and hydrogen gas
Q134: For the equilibrium N2(g)+ 3 H2(g)
Q135: A plot of ln K vs.1/T
Q136: Consider the equilibrium,2 NOBr(g)
Q137: For the reaction of hydrogen and
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents