If 3.50 g of Ni are reacted with excess oxygen to form nickel oxide (NiO) under standard state conditions,what is the entropy change for the reaction? 2 Ni(s) + O2(g) 2 NiO(s) 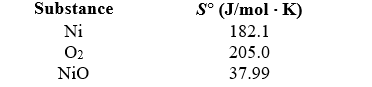
A) -49.3 J/ K
B) -24.7 J/ K
C) -14.7 J/ K
D) +49.3 J/ K
E) -10.4 J/ K
Correct Answer:
Verified
Q17: In a spontaneous process, the entropy of
Q38: During which of the following processes does
Q49: The gas above the liquid in
Q53: When solid ammonium nitrate (NH4NO3) dissolves
Q58: What is the entropy change in
Q62: Indicate which one of the following
Q63: NO gas is converted to NO2
Q64: Which of the following statements regarding free
Q65: Indicate which one of the following
Q66: Determine the standard entropy change of
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents