Indicate which one of the following reactions results in a negative Ssys.
A) H2O(g) H2O(s)
B) CaCO3(s) CaO(s) + CO2(g)
C) CuSO4 5 H2O(s) CuSO4(s) + 5 H2O(g)
D) 14 O2(g) + 3 NH4NO3(s) + C10H22 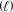 3 N2(g) + 17 H2O(g) + 10 CO2(g)
3 N2(g) + 17 H2O(g) + 10 CO2(g)
E) CO2(aq) CO2(g)
Correct Answer:
Verified
Q38: During which of the following processes does
Q61: If 3.50 g of Ni are
Q62: Indicate which one of the following
Q63: NO gas is converted to NO2
Q64: Which of the following statements regarding free
Q66: Determine the standard entropy change of
Q67: In an experiment,1.000 atm of H2(g)in
Q68: Determine the change in the standard
Q69: What is the standard entropy change when
Q70: Determine
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents