What is the standard entropy change when 10.0 g of methane reacts with 10.0 g of oxygen to form carbon dioxide and liquid water? 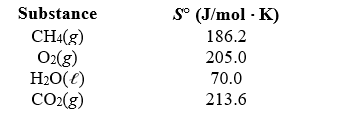
A) -121 J/K
B) -37.9 J/K
C) -243 J/K
D) -154 J/K
E) -16.8 J/K
Correct Answer:
Verified
Q64: Which of the following statements regarding free
Q65: Indicate which one of the following
Q66: Determine the standard entropy change of
Q67: In an experiment,1.000 atm of H2(g)in
Q68: Determine the change in the standard
Q70: Determine
Q71: Determine the entropy change for the
Q72: Which of the relationships between the
Q73: What is the entropy change if
Q74: Determine
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents