In an experiment,1.000 mol of sodium metal is placed in a container and reacted with 4.000 mol of chlorine gas to form sodium chloride under standard state conditions.Determine Srxn given the following. 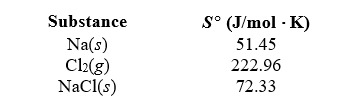
A) -181.2 J/K
B) -90.60 J/ K
C) -724.8 J/K
D) -45.30 J/K
E) -202.1 J/K
Correct Answer:
Verified
Q74: Determine
Q75: Estimate the standard molar entropy of
Q75: Which of the following statements regarding
Q76: Determine
Q77: The enthalpy and entropy of vaporization
Q78: The enthalpy and entropy of vaporization
Q80: The enthalpy and entropy of vaporization
Q81: Methane and water vapor can react
Q82: Estimate the standard free energy change
Q84: Determine the standard free energy change
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents