Multiple Choice
Determine Grxn for C4H10(  ) +
) + 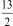 O2(g) 4 CO2(g) + 5 H2O(g) given the following.
O2(g) 4 CO2(g) + 5 H2O(g) given the following. 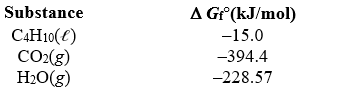
A) -2705.5 kJ/mol
B) -608.0 kJ/mol
C) -1791.0 kJ/mol
D) -2735.5 kJ/mol
E) +608.0 kJ/mol
Correct Answer:
Verified
Related Questions
Q60: At constant T and P, any
Q75: Which of the following statements regarding
Q82: Estimate the standard free energy change
Q84: Determine the standard free energy change
Q85: Calculate the maximum amount of work
Q86: Hydrochloric acid (HCl)reacts with sodium hydroxide
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents