Calculate the maximum amount of work that can be done by the reaction CH4(g) + 2 O2(g) CO2(g) + 2 H2O(g) ,given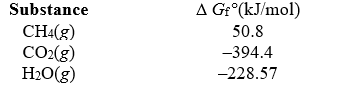
A) -673.8 kJ/mol
B) -750.9 kJ/mol
C) -902.3 kJ/mol
D) -216.6 kJ/mol
E) -800.7 kJ/mol
Correct Answer:
Verified
Q60: At constant T and P, any
Q75: Which of the following statements regarding
Q80: The enthalpy and entropy of vaporization
Q81: Methane and water vapor can react
Q82: Estimate the standard free energy change
Q84: Determine the standard free energy change
Q86: Hydrochloric acid (HCl)reacts with sodium hydroxide
Q87: Determine
Q89: The symbol Q90: At body temperature,many proteins have a![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents