Given the following data,determine the molar free energy of combustion for propane gas,C3H8. 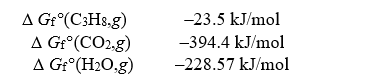
A) -1629.1 kJ/mol
B) -2074.0 kJ/mol
C) -599.5 kJ/mol
D) +599.5 kJ/mol
E) +1582.1 kJ/mol
Correct Answer:
Verified
Q42: Processes are always spontaneous when _
Q89: The symbol Q90: At body temperature,many proteins have a Q91: Consider substances that exist as liquids under Q92: Hydrogen reacts with nitrogen to form Q93: Zinc sulfide can be oxidized by Q96: Hydrogen reacts with nitrogen to form Q98: Given the following data,determine the free energy Q99: The dissolution of ammonium nitrate in water Q99: Determine the value of ![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents