Given the following data,determine the free energy of formation for liquid ethanol,C2H5OH.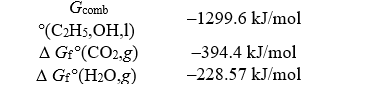
A) -2775.1 kJ/mol
B) -676.6 kJ/mol
C) -174.9 kJ/mol
D) +174.9 kJ/mol
E) +676.6 kJ/mol
Correct Answer:
Verified
Q42: Processes are always spontaneous when _
Q93: Zinc sulfide can be oxidized by
Q94: Given the following data,determine the molar free
Q96: Hydrogen reacts with nitrogen to form
Q99: The dissolution of ammonium nitrate in water
Q99: Determine the value of
Q100: A reaction with a low enthalpy
Q101: Which of the following statements about
Q102: The enthalpy and entropy of vaporization
Q103: Dinitrogen tetroxide (N2O4)decomposes to nitrogen dioxide
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents