A solution is prepared by mixing 50.00 g of methanol (CH3OH,32.04 g/mol) with 50.00 g of ethanol (CH3CH2OH,46.07 g/mol) .Use the following data to determine the vapor pressure of this solution at 20 C. 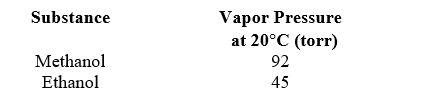
A) 69 torr
B) 57 torr
C) 79 torr
D) 73 torr
E) 83 torr
Correct Answer:
Verified
Q30: Which statement below regarding evaporation is NOT
Q38: Which of the solutions shown here will
Q41: The smell of fresh-cut pine is due
Q42: A solution is prepared by mixing
Q43: You wish to prepare a solution
Q44: A solution is prepared by mixing
Q45: Which of the following regarding the vapor
Q46: A solution is prepared by adding
Q51: The aroma from almonds and cherries is
Q56: Gasoline is primarily a mixture of
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents