A solution is prepared by mixing 75 g of methanol (CH3OH,32.04 g/mol) with 25 g of ethanol (CH3CH2OH,46.07 g/mol) .What is the mole fraction of ethanol in the vapor phase at 20 C? 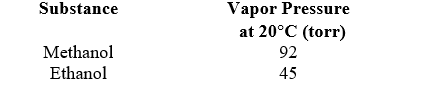
A) 0.10
B) 0.12
C) 0.19
D) 0.32
E) 0.54
Correct Answer:
Verified
Q42: A solution is prepared by mixing
Q43: You wish to prepare a solution
Q44: A solution is prepared by mixing
Q45: Which of the following regarding the vapor
Q46: A solution is prepared by adding
Q48: A solution is prepared by adding
Q49: Which statement regarding nonideal solutions is NOT
Q50: A solution is prepared by mixing
Q51: You must use 168 g of
Q52: What is the vapor pressure of
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents