A solution is prepared by mixing 3.50 mL dichloromethane (CH2Cl2,84.93 g/mol,1.33 g/mL) with 3.50 mL dibromomethane (CH2Br2,173.8 g/mol,2.48 g/mL) .By what factor is the vapor phase enriched in CH2Cl2 at 25 C? 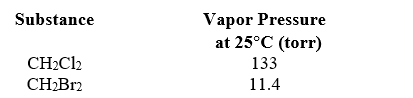
A) 35.3
B) 10.8
C) 2.06
D) 1.77
E) 11.7
Correct Answer:
Verified
Q55: A solution contains 6.50 mol water,
Q56: A solution is prepared by mixing
Q57: A solution is prepared by mixing
Q60: Which of the following pairs of liquids
Q60: A solution is prepared by mixing
Q61: A solution is prepared by dissolving 0.330
Q62: At 25
Q65: Which of the following pairs of liquids
Q69: The vapor pressure of an aqueous
Q95: The normal temperature range of the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents