Which of the following statements regarding the phase diagram of water and an aqueous solution is NOT correct? Temperature is on the x-axis; pressure,on the y-axis. 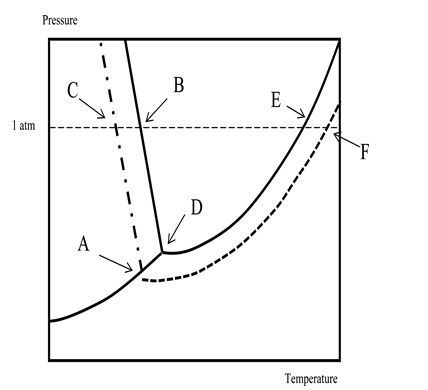
A) The boiling point of the solution is higher than that of the solvent by an amount indicated by the difference in temperature between points E and F.
B) Point D corresponds to the triple point of the solvent.
C) The solution boils at the temperature corresponding to point F.
D) The normal freezing point of the solution corresponds to point A.
E) The freezing point of the solvent is higher than that of the solution.
Correct Answer:
Verified
Q31: Indicate which aqueous solution has the fastest
Q45: Indicate which aqueous solution has the highest
Q52: Indicate which aqueous solution has the lowest
Q66: The concentration unit of molality is symbolized
Q69: The vapor pressure of benzene (C6H6,78.12
Q70: A 4.028 m aqueous ethylene glycol
Q72: How many moles of solute are there
Q74: What is the boiling point of
Q78: What is the boiling point elevation
Q93: At 25
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents