Given the following reactions,what is the overall enthalpy change for the following reaction? C4H4(g) + 2 H2(g) C4H8(g) 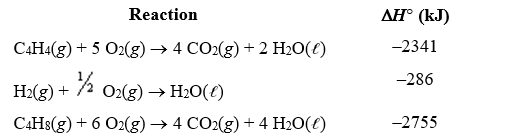
A) +128 kJ
B) -5672 kJ
C) -986 kJ
D) -158 kJ
E) -444 kJ
Correct Answer:
Verified
Q85: Which statement regarding combustion of a sample
Q92: Which statement regarding combustion of a sample
Q113: When a 13.0 g sample of NaOH(s)dissolves
Q114: Isooctane is a good model for gasoline.When
Q115: In an experiment,100.0 g of water at
Q116: When 1.14 g of octane (molar mass
Q118: In terms of the enthalpy of formation,which
Q120: A food sample was burned in a
Q121: The complete combustion of carbon compounds
Q122: Coal is converted into cleaner,more transportable fuels
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents